With over 15 years of computer systems validation experience, we work to create CSV processes that are based on FDA regulations and guidance, best practices, and the characteristics of the system being validated.
Computer systems validation (CSV) is a critical requirement of electronic record and system compliance, as described in the FDA 21 CFR 11.10(a) and EMA Annex 11, Section 4. The validation process is designed to provide a high degree of assurance that both new and existing computer systems will consistently fulfill their intended purpose by producing results which meet predetermined specifications and quality attributes – accuracy, reliability, consistent intended performance, and the ability to discern invalid or altered records.
As scientific applications constantly evolve to keep up with the needs of the people and businesses that use them, Life Science companies must perform validation activities on an ongoing basis in order to reduce compliance risk, ensure quality, and maintain data integrity.
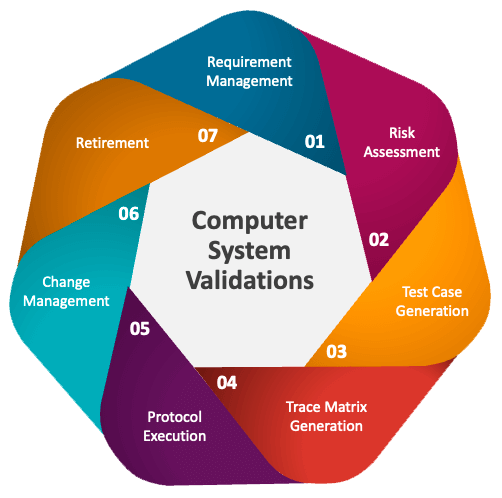
A “Computer System” in an FDA regulated laboratory is more than just computer hardware and software – it also includes any equipment and instruments linked to the system, as well as the trained staff that operate the system and/or equipment using Standard Operating Procedures (SOPs) and manuals. Computer system validation requires a comprehensive set of both static and dynamic testing activities that must be conducted throughout the Software Document Life Cycle (SDLC)
At Elkforce, we are experts in IT risk identification and management along with regulatory compliance. As such, we understand that computer system validation is not a “one size fits all” process. With over 20 years of validation experience, we work to create CSV processes that are based on the latest FDA regulations and guidance (GAMP®5: A Risk-Based Approach to Compliant GxP Computerized Systems), best practices, and the characteristics of the system being validated. Our CSV processes typically involve:

